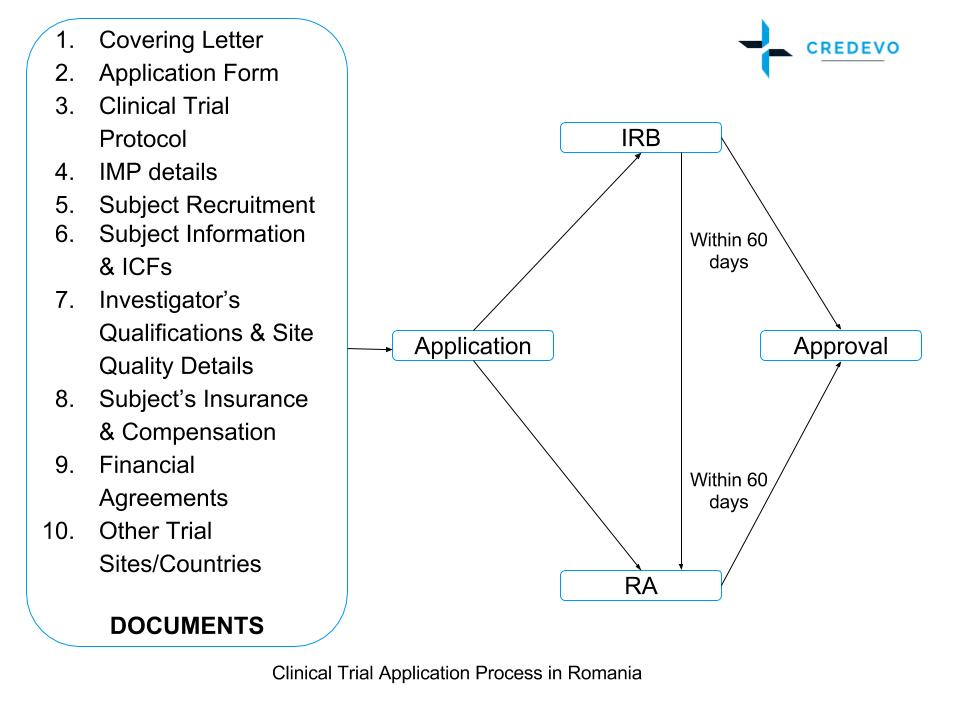
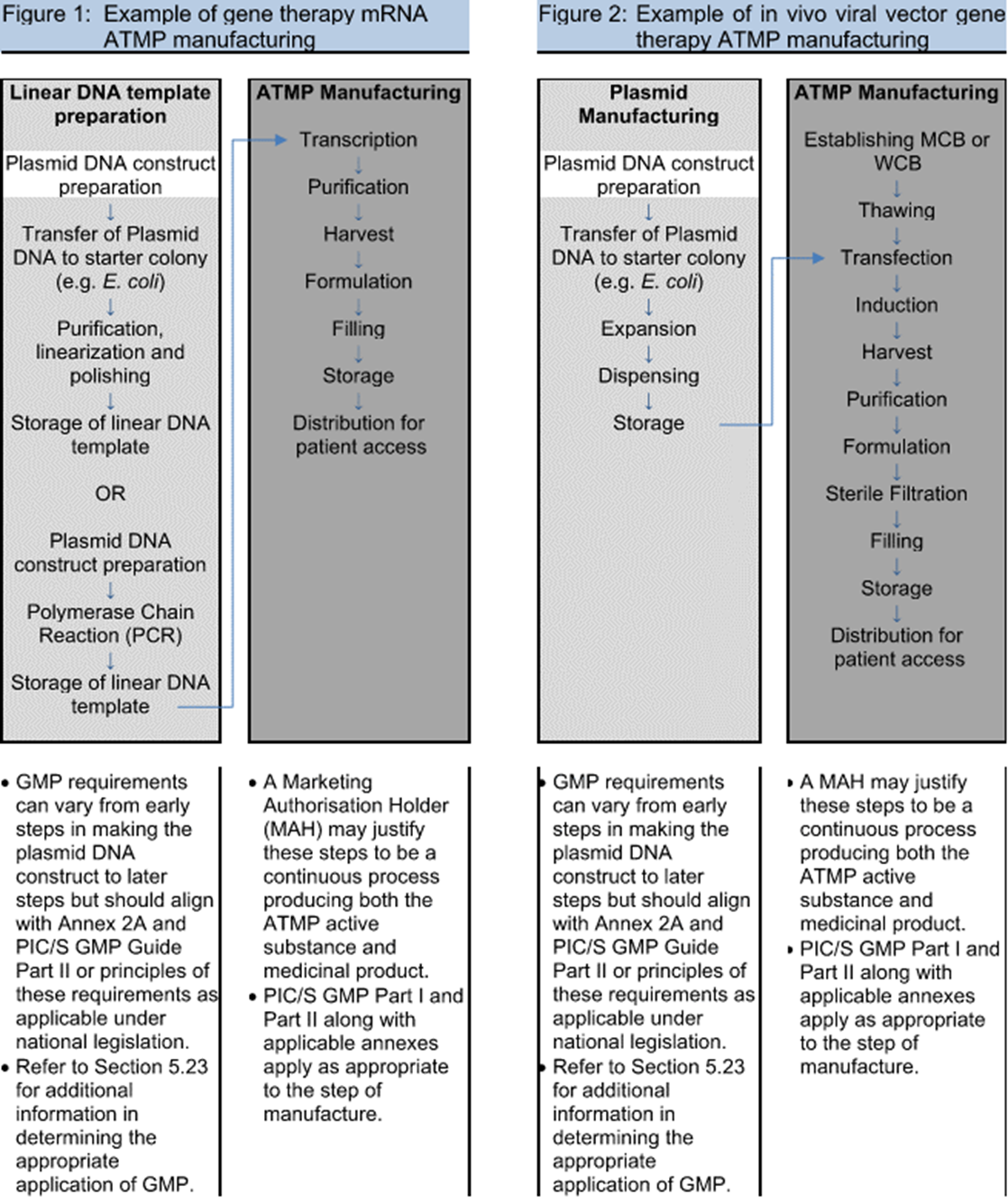
Guidelines on applications for authorisation to conduct toxicological and pharmacological trials for the purpose of assessing th
Annex 1: CLINICAL TRIAL APPLICATION FORM (CTA) To be completed by Applicants for all Clinical Trials Study Title: Protocol No:
Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trial
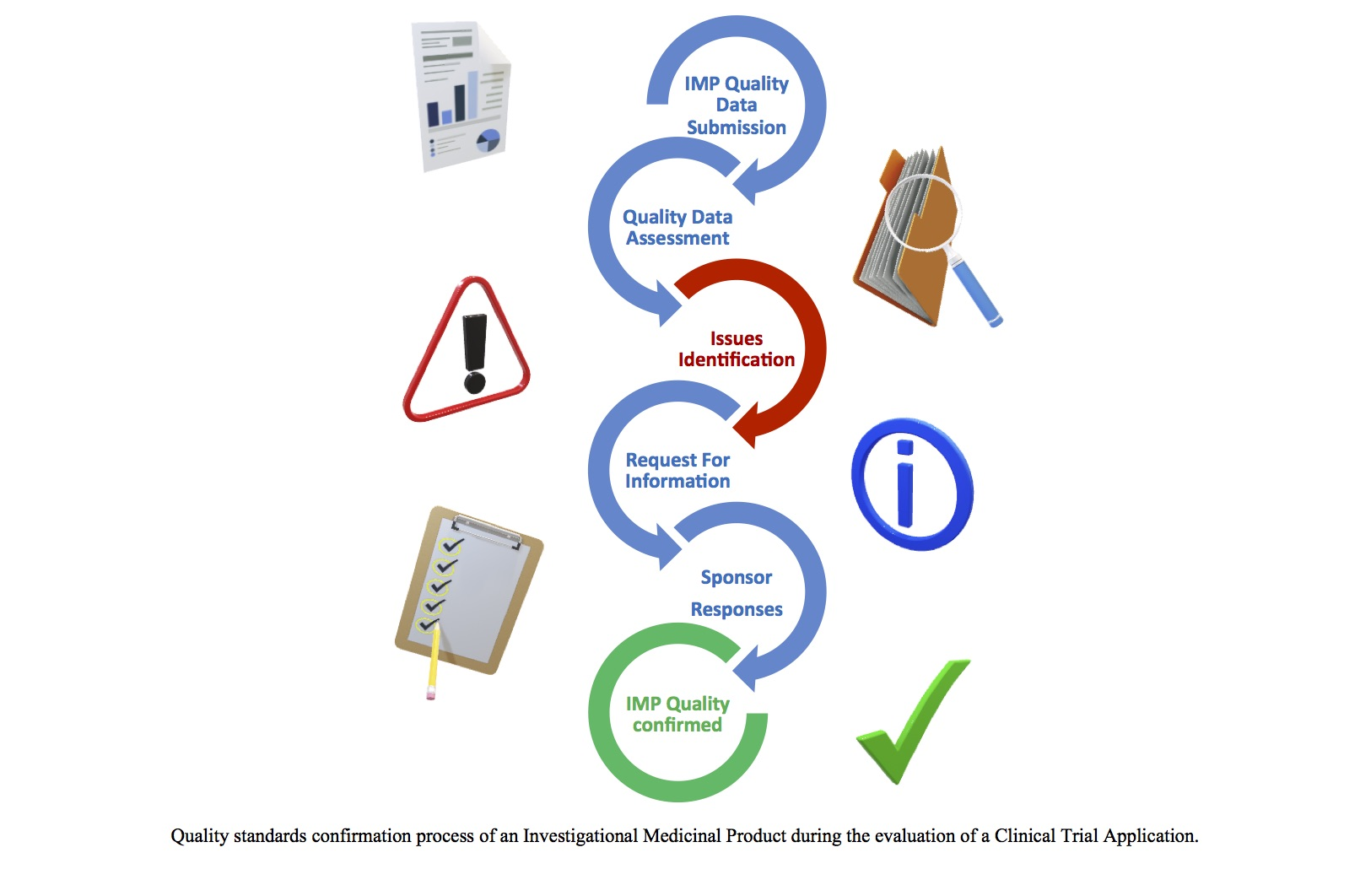
Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML
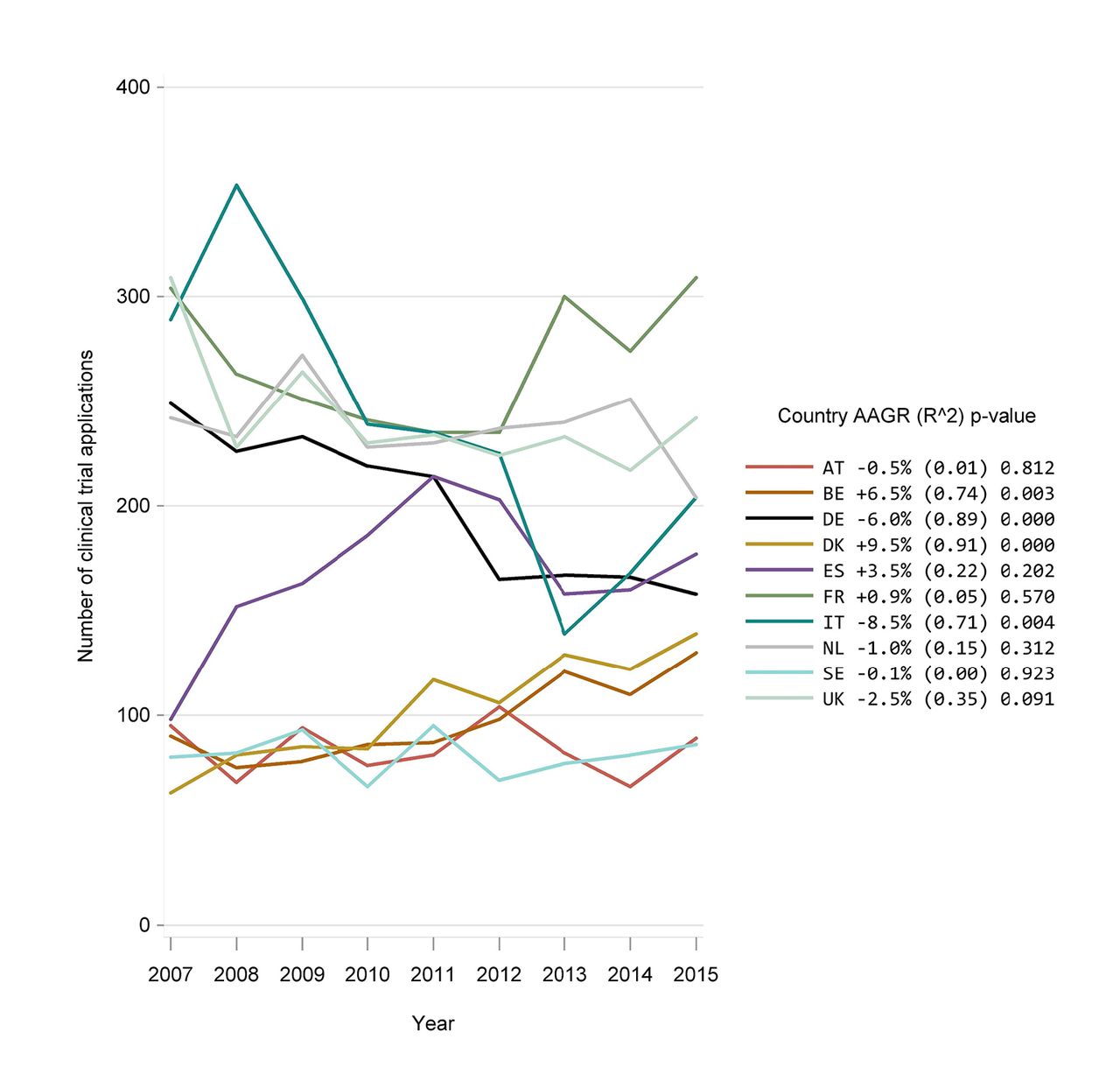
Development in the number of clinical trial applications in Western Europe from 2007 to 2015: retrospective study of data from national competent authorities | BMJ Open

Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents - Journal of Clinical Epidemiology
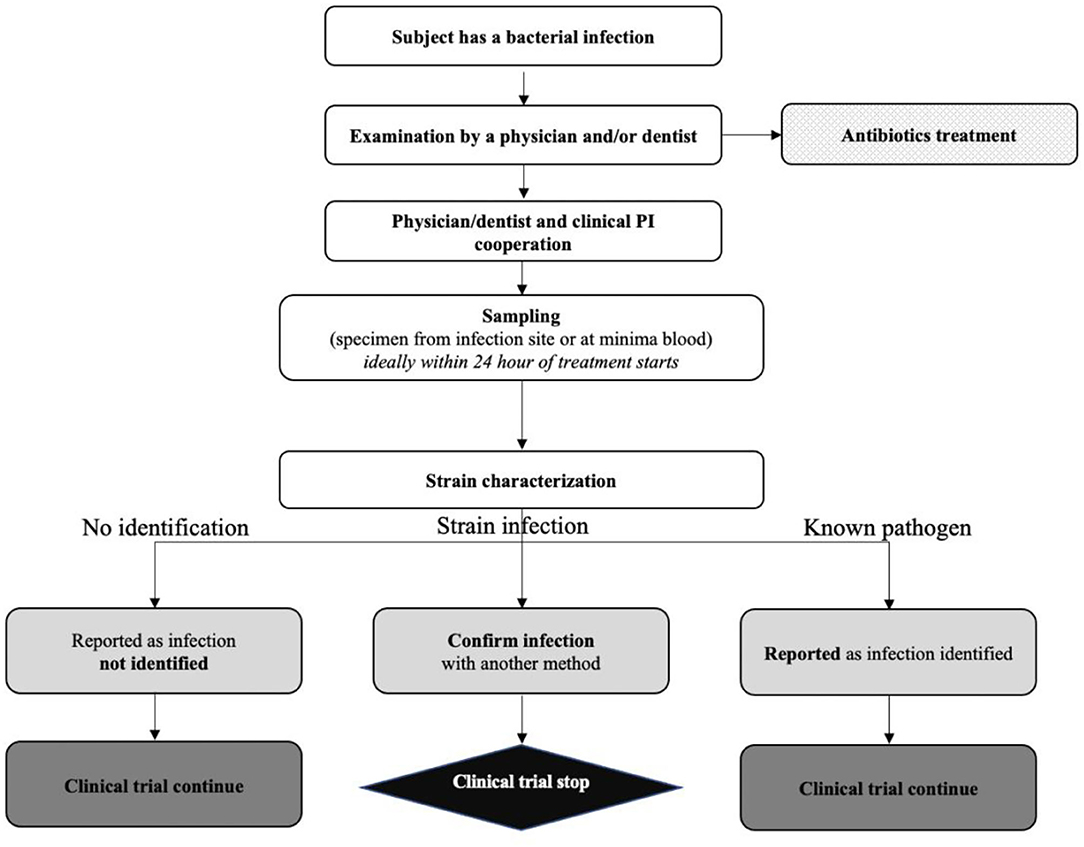
Frontiers | Entering First-in-Human Clinical Study With a Single-Strain Live Biotherapeutic Product: Input and Feedback Gained From the EMA and the FDA
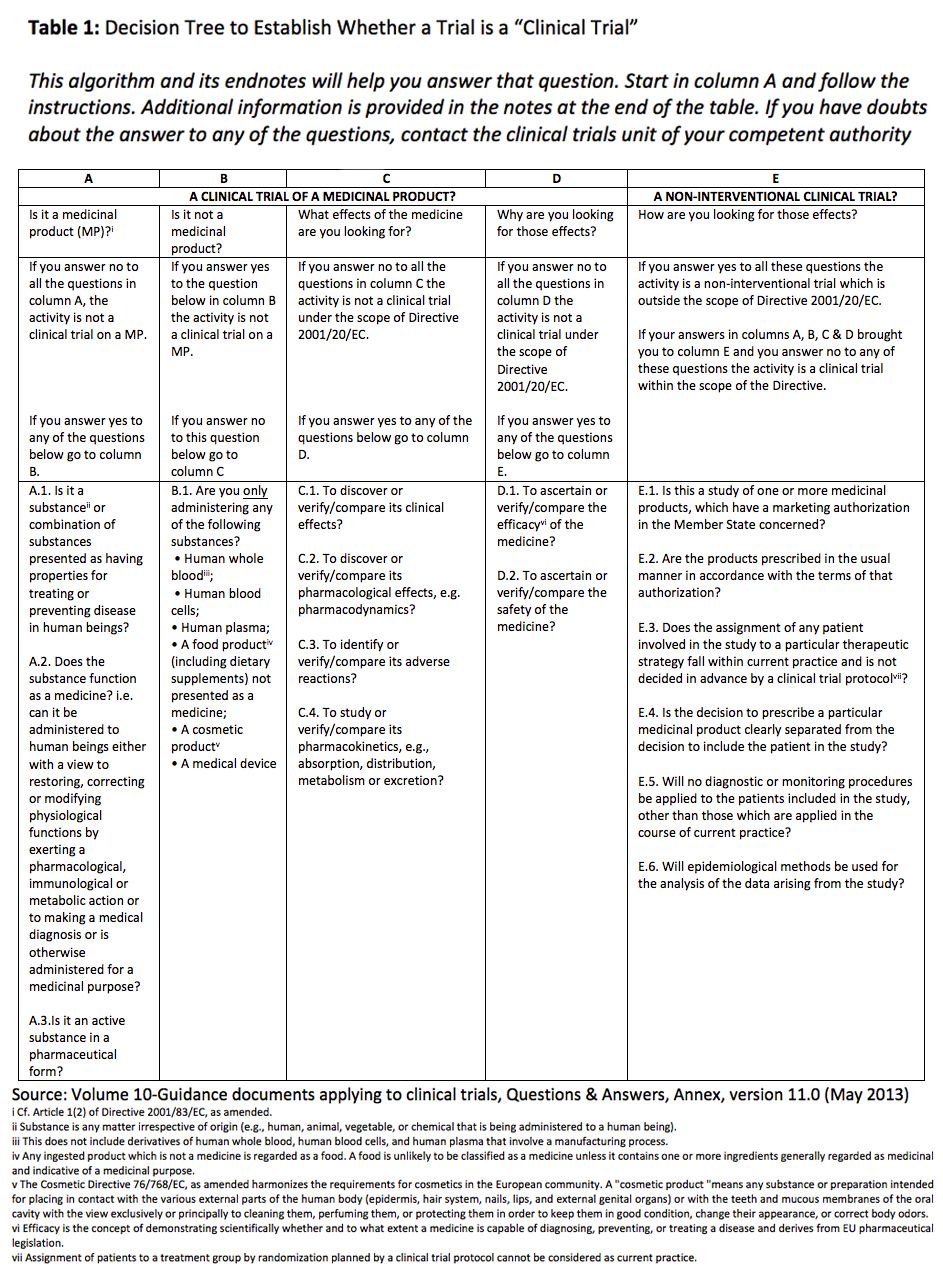
Interventional vs. Non-interventional Study Classification in the EU: Considerations on the Impact of Direct-to-Patient Contacts
Page 1 of 5 MONITORING SERVICES FOR A CLINICAL TRIAL EXP_17_2019 Background The Barcelona Institute for Global Health, ISGlobal

Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device - PDF Free Download