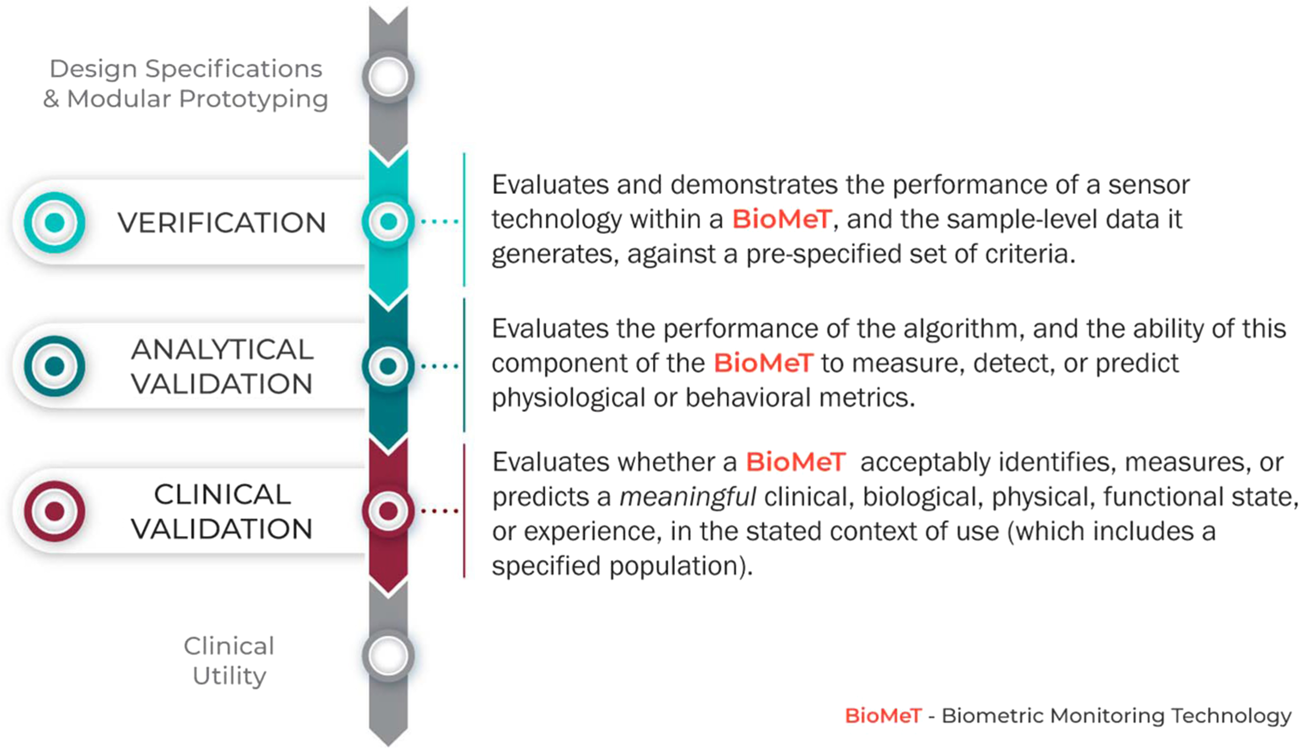
Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs) | npj Digital Medicine

Regulatory oversight of cell therapy in China: Government's efforts in patient access and therapeutic innovation - ScienceDirect

PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials
Welcome and Overview - International Workshop on ethical and GCP aspects of the acceptance of clinical trials submitted in Marketing Authorisation Applications to EMA.