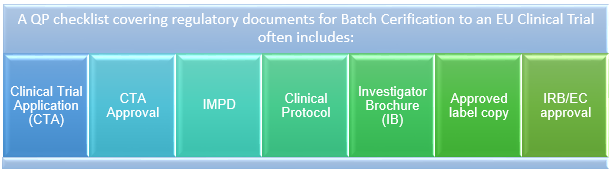
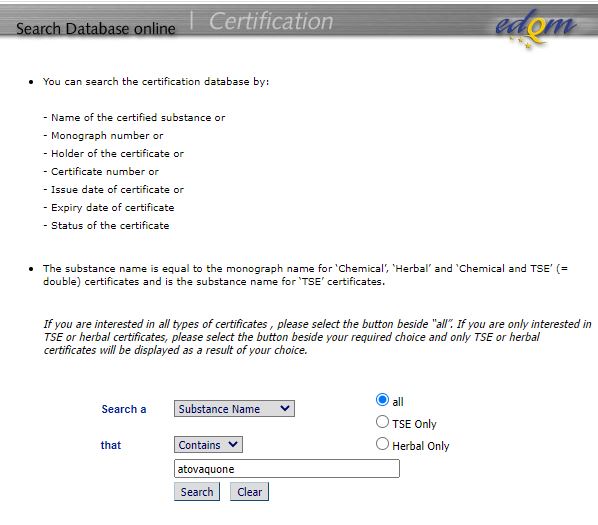
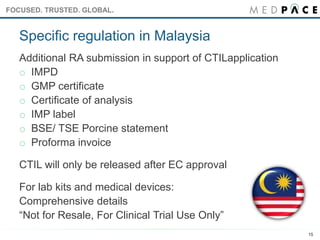
Managing Clinical Trial Application (CTA) Acceptability to Support Phase I Clinical Studies in the United Kingdom

An Overview by Saravanaraja Subramanian 1. Risk & Regulations of TSE/BSE in pharmaceuticals are of great importance because of its irreversible fatal. - ppt download
Clinical trials drugs for USA, Canada, Germany in Ukraine | Phase 1, 2, 3, 4 medical research in Clinical and Diagnostics Center
A comparative analysis of important public clinical trial registries, and a proposal for an interim ideal one | PLOS ONE
National Differences in Requirements for Ethical and Competent Authority Approval for a Multinational Vaccine Trial under the EU
TEMPLATE FOR THE QUALITY ASSESSMENT OF CLINICAL TRIAL APPLICATIONS African Vaccine Regulatory Forum (AVAREF) QUALITY ASSESSMENT
Detailed guidance for the request for authorisation of a clinical trial on a medicinal product for human use to the competent au